Catalytic formation of N3-substituted quinazoline-2,4(1H,3H)-diones by Pd(ii)EN@GO composite and its mechanistic investigations through DFT calculations†
Abstract
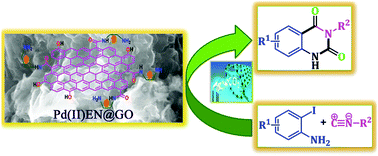
In the current era, the scientific community is very much interested to utilize the greenhouse gas, carbon dioxide, through chemical fixation in order to produce value-based fine organic chemicals. The chemical combination of atmospheric carbon dioxide, isocyanides, and 2-iodoaniline in a one-pot reaction for the synthesis of quinazoline-2,4(1H,3H)-dione derivatives is a straight forward and attractive methodology to avoid multi-step and more toxic reagent containing routes. In this study, a heterogeneous catalyst was designed and synthesized from aminically modified graphene oxide by the incorporation of palladium metal. The catalyst was characterized by FT-IR, XRD, ICP-AES, Raman spectroscopy, XPS, TEM, SEM, EDX, and N2 absorption desorption studies. In this report, the formation of N3-substituted 2,4(1H,3H)-quinazolinediones was performed under mild and heterogeneous reaction conditions under 1 bar CO2 pressure. The catalyst is very efficient to produce the quinazoline derivatives. For the investigation of the mechanistic route of the catalytic reaction, density functional theory (DFT) calculations were also monitored. We have checked the recyclability of the catalyst, the results indicated that the catalyst maintained its catalytic efficacy even after six cycles of use.


 Please wait while we load your content...
Please wait while we load your content...