Enzymatically triggered chromogenic cross-linking agents under physiological conditions†
Abstract
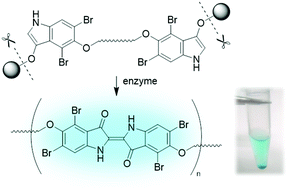
The ability to cross-link molecules upon enzymatic action under physiological conditions holds considerable promise for use in diverse life sciences applications. Here, an enzymatically triggered “click reaction” has been developed by exploiting the longstanding indigo-forming reaction from indoxyl β-glucoside. The covalent cross-linking proceeds in aqueous solution, requires the presence only of an oxidant (e.g., O2), and is readily detectable owing to the blue color of the resulting indigoid dye. To achieve facile indigoid formation in the presence of a bioconjugatable tether, diverse indoxyl β-glucosides were synthesized and studied in enzyme assays with four glucosidases including from tritosomes (derived from hepatic lysosomes) and rat liver homogenates. Altogether 36 new compounds (including 15 target indoxyl-glucosides for enzymatic studies) were prepared and fully characterized in pursuit of four essential requirements: enzyme triggering, facile subsequent indigoid dye formation, bioconjugatability, and synthetic accessibility. The 4,6-dibromo motif in a 5-alkoxy-substituted indoxyl-glucoside was a key design feature for fast and high-yielding indigoid dye formation. Two attractive molecular designs include (1) an indoxyl-glucoside linked to a bicyclo[6.1.0]nonyl (BCN) group for Cu-free click chemistry, and (2) a bis(indoxyl-glucoside). In both cases the linker between the reactive moieties is composed of two short PEG groups and a central triazine derivatized with a sulfobetaine moiety for water solubilization. Glucosidase treatment of the bis(indoxyl-glucoside) in aqueous solution gave oligomers that were characterized by absorption, dynamic light-scattering, and 1H NMR spectroscopy; optical microscopy; mass spectrometry; and HPLC. Key attractions of in situ indigoid dye formation, beyond enzymatic triggering under physiological conditions without exogenous catalysts or reagents, are the chromogenic readout and compatibility with attachment to diverse molecules.


 Please wait while we load your content...
Please wait while we load your content...