Tuning product selectivity in the catalytic oxidation of glycerol by employing metal-ZSM-11 materials†
Abstract
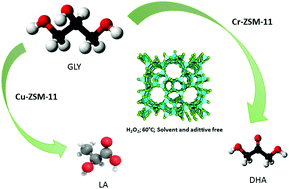
Copper and chromium supported on ZSM-11 (MEL-type structure) microporous zeolites were investigated as catalysts for glycerol oxidation towards dihydroxyacetone and lactic acid. ZSM-11 was synthesized by hydrothermal crystallization. Subsequently, alkaline treatment of desilication was carried out in order to generate zeolites with micro-mesoporosity. Ion exchange with ammonium chloride was performed to recover acidic sites and then, Cr(III) and Cu(II) species were incorporated onto these materials. Finally, thermal treatment at 500 °C was carried out. These materials were characterized by different techniques and then evaluated in the glycerol oxidation reaction by employing H2O2 as an oxidizing agent. The maximum conversion of glycerol (∼70%) was reached over the Cr–ZSM-11 catalyst with a selectivity of ∼28% towards dihydroxyacetone. Meanwhile, Cu-ZSM-11 offered 50% glycerol conversion with a selectivity of 68% towards lactic acid. In both the cases, the optimal reaction conditions were studied to maximize the selectivity towards the products mentioned above.


 Please wait while we load your content...
Please wait while we load your content...