Design and synthesis of a peptide derivative of ametantrone targeting the major groove of the d(GGCGCC)2 palindromic sequence†
Abstract
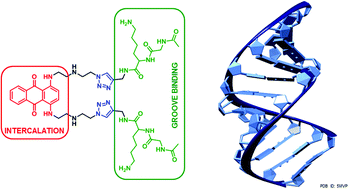
In oncology, some DNA intercalating agents have been used in chemotherapy for years to eradicate cancer cells, but these drugs generally suffer from a lack of selectivity for malignant tissues and consequently induce major side-effects. We report herein the design and synthesis of an antitumor intercalating agent ametantrone complemented with two identical peptide arms including a central Lys residue in order to selectively target palindromic sequences of DNA of malignant cells. The peptide arms are linked to the ametantrone core through 1,2,3-triazole. According to our docking prediction, this compound should be double-stranded β-sheet structured, and it has been designed to interact with two guanine residues upstream from a central d(CpG)2 intercalation site on each DNA strand, owing to the H-bonds involving the Lys terminal side chain ammonium group of the peptide arms. This new ametantrone derivative has been obtained thanks to a convergent synthetic pathway, whose key steps were double nucleophilic substitution performed on the ametantrone core, followed by “double-site” 1,3-dipolar cycloaddition affording the 1,4-disubstituted triazole linker almost quantitatively. Preliminary binding assays performed by mass spectrometry proved its accuracy for DNA palindromic sequences. The cytotoxicity of this compound was evaluated on three cancer cell lines and one healthy cell line, and compared to that of mitoxantone, a dihydroxylated analog of ametantrone. Such a peptide derivative was about ten-fold less cytotoxic than mitoxantrone on these cancer cell lines, but about fifty times less cytotoxic on healthy cells. This study could open new avenues towards the design of targeted intercalating agents.


 Please wait while we load your content...
Please wait while we load your content...