A hybrid ionic liquid-based electrolyte for high-performance lithium–sulfur batteries
Abstract
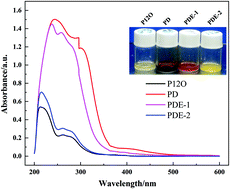
A hybrid ionic liquid (IL)-based electrolyte consisting of N-methoxyethyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide (P1,2O1TFSI) and the support solvent(s) 1,3-dioxolane (DOL) and/or ethyl 1,1,2,2-tetrafluoroethyl ether (ETFE) was applied in lithium–sulfur batteries. It was found that changing the solvent composition greatly influences the overall properties of the electrolyte. The ternary electrolyte P1,2O1TFSI/DOL/ETFE due to the merits of acceptable ion conductivity, moderate polysulfide solubility and good Li compatibility helps the cells to obtain improved cycle stability and rate capability compared to pure IL and the IL/DOL binary system. The synergistic function of two support solvents (DOL and ETFE) with a great difference in donor ability together with IL is considered to establish a reasonable balance between polysulfide dissolution and Li surface protection, thus achieving a controllable shuttle phenomenon and excellent cell performances.


 Please wait while we load your content...
Please wait while we load your content...