Development and validation of a bioanalytical HPLC method for simultaneous estimation of cinnamaldehyde and cinnamic acid in rat plasma: application for pharmacokinetic studies†
Abstract
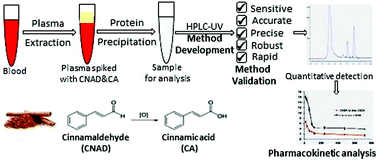
A simple, specific, sensitive and rapid High Performance Liquid Chromatographic (HPLC) method was developed and validated for simultaneous detection of cinnamaldehyde (CNAD) and its metabolite, cinnamic acid (CA), in small volumes of rat plasma. Biological sample preparation involving simple extraction with an organic solvent was adopted to eliminate any chromatographic solvent effects. Chromatographic separation was done using a C18 column using methanol: acetonitrile: 2% glacial acetic acid (20 : 50 : 30, v/v) as the mobile phase at a flow rate of 0.8 ml min−1 and UV detection at 292 nm. The retention times of CA and CNAD were found to be 6 and 7.1 min, respectively. The method was proven to be linear over a plasma concentration range of 0.001 to 1 μg ml−1 with mean correlation coefficients of 0.9993 and 0.9995 for CNAD and CA, respectively. The lower limit of quantification and detection of the newly developed method was determined to be 1.0 ng ml−1 for CNAD and CA. The mean recovery values were found to lie between 95.31 to 118.8%. All the analytes were stable with no significant degradation after repeated freeze-thaw cycles of the processed plasma samples. The method was successfully applied to evaluate pharmacokinetic parameters of CNAD and CA in Wistar albino rats, following a single dose of 10 mg per kg of bw through an intravenous (i.v.) route. Thus, such a simple isocratic elution and one-step precipitation-extraction approach would result in rapid and cost-effective quantification of these analytes from either food or biological samples.


 Please wait while we load your content...
Please wait while we load your content...