The role of oxygen defects in metal oxides for CO2 reduction
Abstract
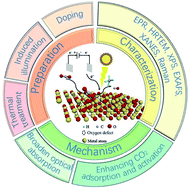
The abuse of fossil fuels release large amount of CO2, causing intense global warming. Using photoreduction and electroreduction to convert CO2 into highly valuable fuels such as CO and CH4 can effectively solve this problem. However, due to the limited activity and selectivity, pristine catalyst materials cannot meet the requirements of practical applications, which means that some modifications to these catalysts are necessary. In this review, a series of research reports on oxygen defect engineering have been introduced. First, the methods of preparing oxygen defects by heat treatment, doping, and photoinduction combined with influencing factors in the preparation are introduced. Subsequently, common characterization methods of oxygen defects including EPR, Raman, XPS, EXAFS, and HRTEM are summarized. Finally, the mechanisms of introducing oxygen defects to improve CO2 reduction are discussed, and include enhancing light absorption, improving CO2 adsorption and activation, as well as promoting stability of the reaction intermediates. The summary of research on oxygen defects provides guidance for researchers who focus on CO2 reduction and accelerate the realization of its industrial applications in the future.
- This article is part of the themed collection: Popular Advances


 Please wait while we load your content...
Please wait while we load your content...