Mechanistic insights into surface contribution towards heat transfer in a nanofluid
Abstract
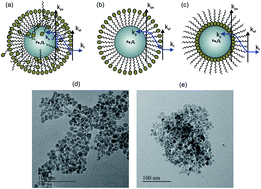
Nanofluids play a very important role in thermal management and heat exchange processes and for a stable nanofluid, a surfactant is a salient material. There are many contrasting reports on the thermal conductivity of nanofluids and the associated heat transport mechanism in nanofluids. In this article, four different types of nanoparticles are synthesized using citric acid and oleic acid as surfactants, followed by the assessment of their thermal conductivities. For a nanofluid of 3 wt% nanoparticles, coated with citric acid in water 67% reduction in thermal conductivity is observed, and on the other hand a 4% enhancement in thermal conductivity is observed for oleic acid-coated nanoparticles in toluene. This anomaly in the thermal transport behaviour of the nanofluid can be related to the surface properties of nanoparticles and the polarity of the base fluid. Theoretical calculation based on molecular dynamics simulations shows that the reduction in long-range interaction and fluid structuration reduce the thermal conductivity in a polar fluid with a polar surfactant coated nanoparticle.


 Please wait while we load your content...
Please wait while we load your content...