Thermally induced intra-molecular transformation and metalation of free-base porphyrin on Au(111) surface steered by surface confinement and ad-atoms†
Abstract
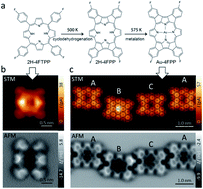
We investigated chemical transformations of a fluorinated free-base porphyrin, 5,10,15,20-tetrakis(4-fluorophenyl)-21,23H-porphyrin (2H-4FTPP) under a Au(111) surface confinement and including gold adatoms by using an experiment and density functional theory based first-principles calculations. Annealing of 2H-4FTPP led to cyclodehydrogenation of the molecule to a π-extended fused aromatic planar compound, 2H-4FPP, and metallation of the porphyrin ring by Au atoms to Au-4FPP complex. Noticeable lowering of bond-dissociation energies of the pyrrole's C–H bonds of the Au(111) supported molecule with respect to their values in the gas phase explained the observed on-surface planarization. Our findings also indicate that Au adatoms may catalyze cleavage of C–H/F bonds in temperature-initiated processes on Au surfaces. BDEs and explicit inclusion of Au adatoms helps to rationalize thermally induced chemical reactions on the respective surface.
- This article is part of the themed collection: Popular Advances


 Please wait while we load your content...
Please wait while we load your content...