Gelation of uranyl ions and gel-derived uranium oxide nanoparticles for gas sensing†
Abstract
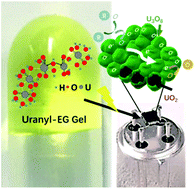
We developed a sol–gel method to synthesize uranium oxide nanoparticles with a clean surface and mixed valences of uranium at the surface. Uranyl gel was formed in ethylene glycol without incorporating any organic gelator and was readily converted to uranium dioxide nanoparticles with uniform size via microwave treatment. The as-prepared uranyl gel showed a high storage modulus of 0.48 kPa. The formation of the gel skeleton benefits from interlinkage of uranyl ions, which was revealed by UV-Vis spectroscopy and X-ray absorption. The U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oax bond was elongated by 0.1 Å and the U–Oeq bond was shortened by 0.25 Å by the gelation. The gel showed thixotropic and self-healing properties owing to the soft connection in the gel skeleton and photo-response attributed to the photo-reduction reaction between uranyl ions and matrix solvent. With the great inclusion properties, the uranyl gel was decomposed by microwave treatment into uranium dioxide nanoparticles with a size of ∼4 nm. The resultant UO2 nanoparticles were easily oxidized in air, and thus presented an n-type semiconductor behaviour and sensitivity to both oxidative and reductive gases such as NO2, EtOH, CO, and NH3.
Oax bond was elongated by 0.1 Å and the U–Oeq bond was shortened by 0.25 Å by the gelation. The gel showed thixotropic and self-healing properties owing to the soft connection in the gel skeleton and photo-response attributed to the photo-reduction reaction between uranyl ions and matrix solvent. With the great inclusion properties, the uranyl gel was decomposed by microwave treatment into uranium dioxide nanoparticles with a size of ∼4 nm. The resultant UO2 nanoparticles were easily oxidized in air, and thus presented an n-type semiconductor behaviour and sensitivity to both oxidative and reductive gases such as NO2, EtOH, CO, and NH3.
- This article is part of the themed collection: Gas sensing


 Please wait while we load your content...
Please wait while we load your content...