On the formation of superoxide radicals on colloidal ATiO3 (A = Sr and Ba) nanocrystal surfaces†
Abstract
Controlling the surface chemistry of colloidal semiconductor nanocrystals is critical to exploiting their rich electronic structures for various technologies. We recently demonstrated that the hydrothermal synthesis of colloidal nanocrystals of SrTiO3, a technologically-relevant electronic material, provided a strong negative correlation between the presence of an O2-related surface defect and hydrazine hydrate [W. L. Harrigan, S. E. Michaud, K. A. Lehuta, and K. R. Kittilstved, Chem. Mater., 2016, 28(2), 430]. When hydrazine hydrate is omitted during the aerobic hydrothermal synthesis, the surface defect is observed. However, it can be removed by either the addition of hydrazine hydrate or by purging the reaction solution with argon gas before the hydrothermal synthesis. We also propose that the formation of the O2-related defect is mediated by the reduction of dissolved O2 by lactate anions that are present from the titanium precursor. This work helps elucidate the nature of the O2-related defect as a superoxide anion 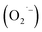 and presents a mechanism to explain its formation during the hydrothermal synthesis of SrTiO3 and related BaTiO3 nanocrystals.
and presents a mechanism to explain its formation during the hydrothermal synthesis of SrTiO3 and related BaTiO3 nanocrystals.



 Please wait while we load your content...
Please wait while we load your content...