Does gold behaves as hydrogen? A joint theoretical and experimental study†
Abstract
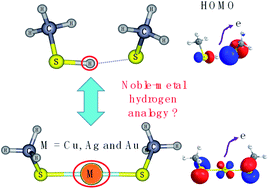
It has been established that the noble-metal–H analogue has been found in a large number of noble-metal–ligand clusters in view of geometric and electronic structures. Here, we demonstrated a different view of noble-metal–H analogue between noble-metal and hydrogen in M(SCH3)2− (M = Cu, Ag, Au and H) systems. Although H(SCH3)2− is a typical ion-hydrogen bonding cluster dramatically different from the chemical bonding clusters of M(SCH3)2− (M = Cu, Ag and Au), the comparison of the two typical bonding patterns has not yet been fully investigated. Through a series of chemical bonding analyses, it is indicated that the evolution has been exhibited from typical ionic bonding in Cu(SCH3)2− to a significant covalent bonding nature in Au(SCH3)2− and hydrogen bonding dominating in H(SCH3)2−. The comparison of M(SCH3)2− (M = Cu, Ag and Au) with H(SCH3)2− illustrates the differences in bonding between noble metals and hydrogen, which are mainly related to their diverse atomic orbitals participating in chemical bonding.

 Please wait while we load your content...
Please wait while we load your content...