Charge transfer between lead halide perovskite nanocrystals and single-walled carbon nanotubes†
Abstract
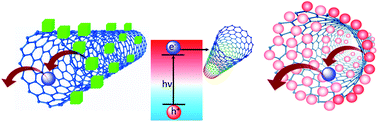
A charge transfer study between lead halide-based perovskite nanocrystals and single-walled carbon nanotubes (PNC@CNT nanocomposite) was performed. Solution-processed MAPbX3 PNCs displayed very bright luminescence, but it quenched in the presence of CNTs. This was attributed to the electron transfer from PNCs to CNTs. The detailed changes in fluorescence lifetime were investigated through time-correlated single-photon counting (TCSPC), which suggested mixed static and dynamic quenching along with a decrease in the lifetime. Morphological changes were investigated via transmission electron microscopy (TEM) and attributed to the incorporation of PNCs on long CNTs. Also, the PNC@CNT nanocomposite was explored for photoinduced current response, which indicated an ∼3 fold increase in photoconductivity under light illumination (with a 1 mV bias). This electron transfer study between PNCs and CNTs contributes to the exploration of charge dynamics.


 Please wait while we load your content...
Please wait while we load your content...