Understanding the driving forces of camptothecin interactions on the surface of nanocomposites based on graphene oxide decorated with silica nanoparticles
Abstract
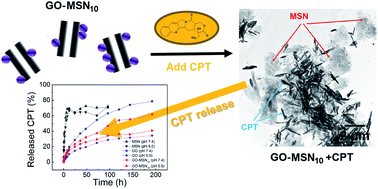
Camptothecin (CPT) is a potent antitumor drug frequently used in studies of drug delivery systems. The poor water solubility and unfavourable pharmacokinetic conditions of CPT and the development of nanomaterials such as mesoporous silica nanoparticles (MSNs), graphene oxide (GO) and a new family of GO decorated with MSNs (GO-MSNs) motivated the present work, which sought to solve these challenges. In this context, release assays showed rapid and prolonged release, respectively, by silica and GO/GO-MSN nanomaterials; release was faster at pH 7.4 and slower at pH 5.0 in all situations. In particular, GO-MSNs presented an important advantage compared to GO due to their slower drug release at pH 7.4 (physiological conditions in blood; slowest release is expected under these conditions) and faster drug delivery at pH 5.0 (acidic conditions in endosomes of cancer cells; fastest release is expected under these conditions). The results, therefore, present the GO-MSN nanomaterial as a potential candidate for antitumor applications. The main drug–nanocarrier chemical interactions (London forces, hydrogen bonds, and electrostatic and dipole–dipole interactions) are also exhaustively described in order to understand the observed differences in drug delivery properties among these nanomaterials and to comprehend the influence of pH on concomitant and dynamic interactions.


 Please wait while we load your content...
Please wait while we load your content...