pH Transitions and electrochemical behavior during the synthesis of iron oxide nanoparticles with gas-diffusion electrodes†
Abstract
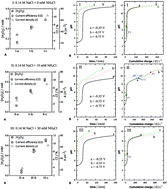
Gas diffusion electrocrystallization (GDEx) was explored for the synthesis of iron oxide nanoparticles (IONPs). A gas-diffusion cathode was employed to reduce oxygen, producing hydroxyl ions (OH−) and oxidants (H2O2 and HO2−), which acted as reactive intermediates for the formation of stable IONPs. The IONPs were mainly composed of pure magnetite. However, their composition strongly depended on the presence of a weak acid, i.e., ammonium chloride (NH4Cl), and on the applied electrode potential. Pure magnetite was obtained due to the simultaneous action of H2O2 and the buffer capacity of the added NH4Cl. Magnetite and goethite were identified as products under different operating conditions. The presence of NH4Cl facilitated an acid–base reaction and, in some cases, led to cathodic deprotonation, forming a surplus of hydrogen peroxide, while adding the weak acid promoted gradual changes in the pH by slightly enhancing H2O2 production when increasing the applied potential. This also resulted in smaller average crystallite sizes as follows: 20.3 ± 0.6 at −0.350 V, 14.7 ± 2.1 at −0.550 and 12.0 ± 2.0 at −0.750 V. GDEx is also demonstrated to be a green, effective, and efficient cathodic process to recover soluble iron to IONPs, being capable of removing >99% of the iron initially present in the solution.


 Please wait while we load your content...
Please wait while we load your content...