Metal oxide decorated porous carbons from controlled calcination of a metal–organic framework†
Abstract
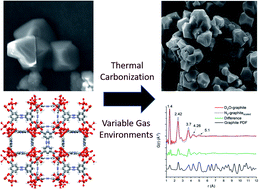
Thermal decomposition of an iron-based MOF was conducted under controlled gas environments to understand the resulting porous carbon structure. Different phases and crystallite sizes of iron oxide are produced based on the specific gas species. In particular, air resulted in iron(III) oxide, and D2O and CO2 resulted in the mixed valent iron(II,III) oxide. Performing the carbonization under non-oxidative or reducing conditions (N2, He, H2) resulted in the formation of a mixture of both iron(II,III) oxide and iron(III) oxide. Based on in situ and air-free handling experiments, it was observed that this is partially due to the formation of zero-valent iron metal that is rapidly oxidized when exposed to air. Neutron pair distribution function analysis provided insight into the effect of the gas environment on the local structure of the porous carbon, indicating a noticeable change in local order between the D2O and the N2 calcined samples.


 Please wait while we load your content...
Please wait while we load your content...