Three dimensional Ni3S2 nanorod arrays as multifunctional electrodes for electrochemical energy storage and conversion applications†
Abstract
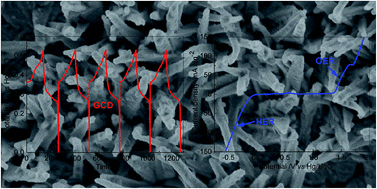
The increasing demand for energy and environmental protection has stimulated intensive interest in fundamental research and practical applications. Nickel dichalcogenides (Ni3S2, NiS, Ni3Se2, NiSe, etc.) are promising materials for high-performance electrochemical energy storage and conversion applications. Herein, 3D Ni3S2 nanorod arrays are fabricated on Ni foam by a facile solvothermal route. The optimized Ni3S2/Ni foam electrode displays an areal capacity of 1602 µA h cm−2 at 5 mA cm−2, excellent rate capability and cycling stability. Besides, 3D Ni3S2 nanorod arrays as electrode materials exhibit outstanding performances for the overall water splitting reaction. In particular, the 3D Ni3S2 nanorod array electrode is shown to be a high-performance water electrolyzer with a cell voltage of 1.63 V at a current density of 10 mA cm−2 for overall water splitting. Therefore, the results demonstrate a promising multifunctional 3D electrode material for electrochemical energy storage and conversion applications.


 Please wait while we load your content...
Please wait while we load your content...