High-performance thermoelectric silver selenide thin films cation exchanged from a copper selenide template†
Abstract
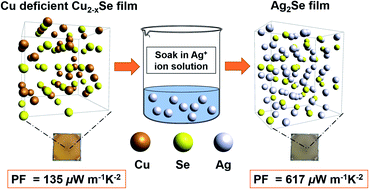
Over the past decade, Ag2Se has attracted increasing attention due to its potentially excellent thermoelectric (TE) performance as an n-type semiconductor. It has been considered a promising alternative to Bi–Te alloys and other commonly used yet toxic and/or expensive TE materials. To optimize the TE performance of Ag2Se, recent research has focused on fabricating nanosized Ag2Se. However, synthesizing Ag2Se nanoparticles involves energy-intensive and time-consuming techniques with poor yield of final product. In this work, we report a low-cost, solution-processed approach that enables the formation of Ag2Se thin films from Cu2−xSe template films via cation exchange at room temperature. Our simple two-step method involves fabricating Cu2−xSe thin films by the thiol-amine dissolution of bulk Cu2Se, followed by soaking Cu2−xSe films in AgNO3 solution and annealing to form Ag2Se. We report an average power factor (PF) of 617 ± 82 μW m−1 K−2 and a corresponding ZT value of 0.35 at room temperature. We obtained a maximum PF of 825 μW m−1 K−2 and a ZT value of 0.46 at room temperature for our best-performing Ag2Se thin-film after soaking for 5 minutes. These high PFs have been achieved via full solution processing without hot-pressing.
- This article is part of the themed collection: Editor’s Choice: Thermoelectric nanostructures


 Please wait while we load your content...
Please wait while we load your content...