On the propagation of the OH radical produced by Cu-amyloid beta peptide model complexes. Insight from molecular modelling†‡
Abstract
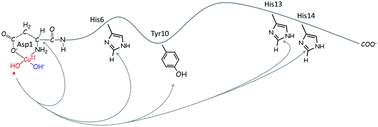
Oxidative stress and metal dyshomeostasis are considered as crucial factors in the pathogenesis of Alzheimer's disease (AD). Indeed, transition metal ions such as Cu(II) can generate Reactive Oxygen Species (ROS) via O2 Fenton-like reduction, catalyzed by Cu(II) coordinated to the Amyloid beta (Aβ) peptide. Despite intensive effort, the mechanisms of ROS-induced molecular damage remain poorly understood. In the present paper, we investigate on the basis of molecular modelling computations the mechanism of OH radical propagation toward the Aβ peptide, starting from the end-product of OH radical generation by Cu(II)·Aβ. We evaluate (i) the OH oxidative capacity, as well as the energetics of the possible Aβ oxidation target residues, by quantum chemistry Density Functional Theory (DFT) on coordination models of Cu(II)/OH/Aβ and (ii) the motion of the OH˙ approaching the Aβ target residues by classical Molecular Dynamics (MD) on the full peptide Cu(II)/OH/Aβ(1–16). The results show that the oxidative capacity of OH coordinated Cu(II)Aβ is significantly lower than that of the free OH radical and that propagation toward Aβ Asp and His residues is favoured over Tyr residues. These results are discussed on the basis of the recent literature on in vitro Aβ metal-catalyzed oxidation and on the possible implications for the AD oxidative stress mechanism.


 Please wait while we load your content...
Please wait while we load your content...