Interplays between copper and Mycobacterium tuberculosis GroEL1†
Abstract
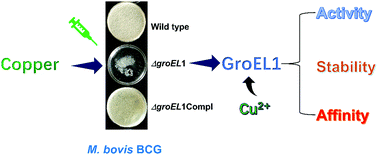
The recalcitrance of pathogenic Mycobacterium tuberculosis, the agent of tuberculosis, to eradication is due to various factors allowing bacteria to escape from stress situations. The mycobacterial chaperone GroEL1, overproduced after macrophage entry and under oxidative stress, could be one of these key players. We previously reported that GroEL1 is necessary for the biosynthesis of phthiocerol dimycocerosate, a virulence-associated lipid and for reducing antibiotic susceptibility. In the present study, we showed that GroEL1, bearing a unique C-terminal histidine-rich region, is required for copper tolerance during Mycobacterium bovis BCG biofilm growth. Mass spectrometry analysis demonstrated that GroEL1 displays high affinity for copper ions, especially at its C-terminal histidine-rich region. Furthermore, the binding of copper protects GroEL1 from destabilization and increases GroEL1 ATPase activity. Altogether, these findings suggest that GroEL1 could counteract copper toxicity, notably in the macrophage phagosome, and further emphasizes that M. tuberculosis GroEL1 could be an interesting antitubercular target.


 Please wait while we load your content...
Please wait while we load your content...