“Nano-lymphatic” photocatalytic water-splitting for relieving tumor interstitial fluid pressure and achieving hydrodynamic therapy†
Abstract
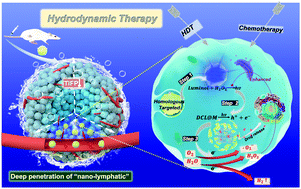
The advancement of anti-tumor nanomedicines is restricted by the delivery efficiency owing to a lack of insight into the intratumoral delivery mechanism, especially traversing the biological barriers. Herein, we explore a new strategy focusing on controlling excessive high tumor interstitial fluid pressure (TIFP) caused by lymphatic lack, which hampers the convective diffusion of nanomedicines to the tumor center. We construct a “nano-lymphatic” system (DOX/g-C3N4/luminol@cytomembrane, DCL@M) based on graphitic carbon nitride (g-C3N4) for photocatalytic water splitting, which can decrease the volume of the tumor interstitial fluid to ameliorate the transfer resistance derived from the high TIFP. In the tumor microenvironment, lactic acid as a sacrificial agent in catalysis greatly promoted the water-splitting efficiency; thus, TIFP in the tumor tissue was reduced to 62.11%, causing an enhancement in blood perfusion to the tumor. The accumulation of the “nano-lymphatic” system (16.73%) in the tumor was 15.9- and 3.31-fold greater than those of free doxorubicin hydrochloride (DOX, 1.05%) and DOX/g-C3N4@cytomembrane (DC@M, 3.03%), respectively. The penetration efficiency of the “nano-lymphatic” system was 8.98% at the tumor center. The “nano-lymphatic” strategy produced reactive oxygen species associated with water splitting to suppress the tumor growth; thus, we define the strategy as hydrodynamic therapy, which provides a new avenue for nanomedicines in traversing biological barriers and achieves a better therapeutic effect.


 Please wait while we load your content...
Please wait while we load your content...