Increased binding of thiophene-based ligands to mercury(ii) with water solubilizing functional groups†
Abstract
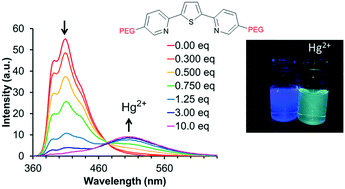
Although the soft sulfur atom of thiophene could selectively coordinate to soft Lewis acids such as mercury(II), sulfur-coordination of thiophene has rarely been observed. The effect of electron donating group and position on the interaction of mercury(II) with thiophene is investigated for a set of dipyridylthiophene-based ligands. The influence on the optical response of ligands functionalized at either the 3,4-position of the thiophene or at the 3-position of the distal pyridines with alcohol, methoxy, octaethylene glycol monomethyl ether, or amine groups in the presence of mercury(II) was investigated. These groups increase both the water solubility of the ligands and optical sensitivity of these ligands in response to mercury(II). These ligands show a ratiometric optical response to mercury(II) in mixed aqueous media as well as in organic solvent. Notably increasing concentrations of mercury(II) result in several distinct products dependent on the concentration of mercury(II). The addition of water solubilizing functional groups also red-shifts the absorption and emission spectra further into the visible region. Herein, we report on the photophysical properties of these ligands in aqueous media and organic solvents, and the change in emission and absorption in response to mercury(II).
- This article is part of the themed collection: Molecular systems for sensing


 Please wait while we load your content...
Please wait while we load your content...