Pharmacophore-based screening of diamidine small molecule inhibitors for protein arginine methyltransferases†
Abstract
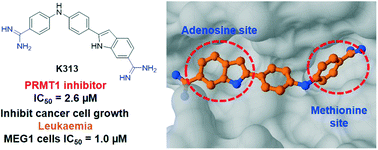
Protein arginine methyltransferases (PRMTs) are essential epigenetic and post-translational regulators in eukaryotic organisms. Dysregulation of PRMTs is intimately related to multiple types of human diseases, particularly cancer. Based on the previously reported PRMT1 inhibitors bearing the diamidine pharmacophore, we performed virtual screening to identify additional amidine-associated structural analogs. Subsequent enzymatic tests and characterization led to the discovery of a top lead K313 (2-(4-((4-carbamimidoylphenyl)amino)phenyl)-1H-indole-6-carboximidamide), which possessed low-micromolar potency with biochemical IC50 of 2.6 μM for human PRMT1. Limited selectivity was observed over some other PRMT isoforms such as CARM1 and PRMT7. Molecular modeling and inhibition pattern studies suggest that K313 is a nonclassic noncompetitive inhibitor to PRMT1. K313 significantly inhibited cell proliferation and reduced the arginine asymmetric dimethylation level in the leukaemia cancer cells.


 Please wait while we load your content...
Please wait while we load your content...