Structure–activity relationship studies on 2,5,6-trisubstituted benzimidazoles targeting Mtb-FtsZ as antitubercular agents†
Abstract
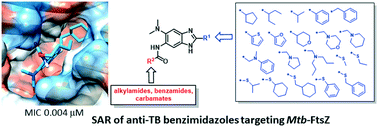
Filamenting temperature sensitive protein Z (FtsZ) is an essential bacterial cell division protein and a promising target for the development of new antibacterial therapeutics. As a part of our ongoing SAR studies on 2,5,6-trisubstituted benzimidazoles as antitubercular agents targeting Mtb-FtsZ, a new library of compounds with modifications at the 2 position was designed, synthesized and evaluated for their activity against Mtb-H37Rv. This new library of trisubstituted benzimidazoles exhibited MIC values in the range of 0.004–50 μg mL−1. Compounds 6b, 6c, 20f and 20g showed excellent growth inhibitory activities ranging from 0.004–0.08 μg mL−1. This SAR study has led to the discovery of a remarkably potent compound 20g (MIC 0.0039 μg mL−1; normalized MIC 0.015 μg mL−1). Our 3DQSAR model predicted 20g as the most potent compound in the library.
- This article is part of the themed collection: Tuberculosis


 Please wait while we load your content...
Please wait while we load your content...