Synthesis and biological screening of a library of macamides as TNF-α inhibitors†
Abstract
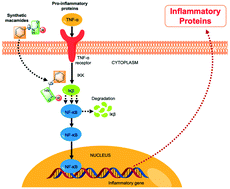
Thirty-five macamide analogues were synthesised by modifying the initial molecular structure. The resulting structures were confirmed using NMR and MS. Cytotoxicity and the anti-inflammatory activity of these synthetic macamides were evaluated in the THP-1 cell line. Preliminary biological evaluation indicated that most of these synthetic macamides did not present cytotoxicity (MTT assay) in the tested cell line with respect to the control (actinomycin D). Regarding the anti-inflammatory activity, several analogues had a greater potential for inhibition of TNF-α than natural macamides. Synthetic macamide 4a was the most active (IC50 = 0.009 ± 0.001 μM) compared to the C87 (control). Through looking at the link between the chemical structure and the activity, our study proves that changes made to natural macamides at the level of the alkyl chain, the benzyl position, the amide bond, and the addition of two methyl groups to the aromatic ring (meta position) lead us to obtaining new macamides with greater anti-inflammatory activity.


 Please wait while we load your content...
Please wait while we load your content...