Synthesis of quinone imine and sulphur-containing compounds with antitumor and trypanocidal activities: redox and biological implications†
Abstract
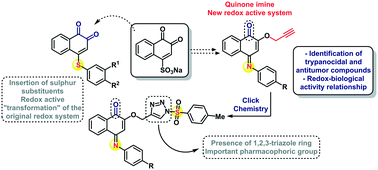
Ortho-Quinones represent a special class of redox active compounds associated with a spectrum of pronounced biological activities, including selective cytotoxicity and antimicrobial actions. The modification of the quinone ring by simple nitrogen and sulphur substitutions leads to several new classes of compounds with their own, distinct redox behaviour and equally distinct activities against cancer cell lines and Trypanosoma cruzi. Some of the compounds investigated show activity against T. cruzi at concentrations of 24.3 and 65.6 μM with a selectivity index of around 1. These results demonstrate that simple chemical modifications on the ortho-quinone ring system, in particular, by heteroatoms such as nitrogen and sulphur, transform these simple redox molecules into powerful cytotoxic agents with considerable “potential”, not only in synthesis and electrochemistry, but also, in a broader sense, in health sciences.
- This article is part of the themed collection: Celebrating Latin American Talent in Chemistry


 Please wait while we load your content...
Please wait while we load your content...