Synthesis and anti-HBV activity of carbocyclic nucleoside hybrids with salient features of entecavir and aristeromycin†
Abstract
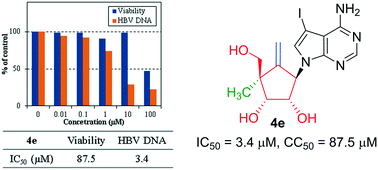
Modified carbocyclic nucleosides (4a–g) constituting 7-deazapurine, 4′-methyl, exocyclic double bond and 2′,3′-hydroxy were synthesized. NOE and X-ray studies of 4c confirmed the α-configuration of 4′-methyl. The anti-HBV assay demonstrated 4e (IC50 = 3.4 μM) without notable cytotoxicity (CC50 = 87.5 μM) as a promising lead for future exploration.


 Please wait while we load your content...
Please wait while we load your content...