Synthesis and 18F-radiolabeling of thymidine AMBF3 conjugates†
Abstract
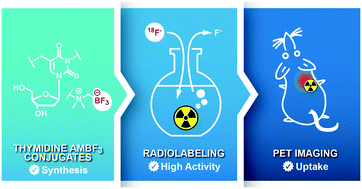
In pursuit of 18F-labeled nucleosides for positron emission tomography (PET) imaging, we report on the chemical and radiochemical synthesis of two thymidine (dT) analogs, dT-C5-AMBF3 and dT-N3-AMBF3, that are radiofluorinated by isotope exchange (IEX) and studied as PET imaging agents in mice with tumor xenografts. dT-C5-AMBF3 shows preferential, and tumor-specific, uptake over dT-N3-AMBF3. This work provides a new synthetic method in order to access new nucleoside tracers for PET imaging.


 Please wait while we load your content...
Please wait while we load your content...