Synthesis, evaluation and molecular modelling of piceatannol analogues as arginase inhibitors†
Abstract
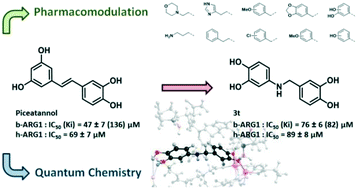
Arginase is involved in a wide range of pathologies including cardiovascular diseases and infectious diseases whilst it is also a promising target to improve cancer immunotherapy. To date, only a limited number of inhibitors of arginase have been reported. Natural polyphenols, among them piceatannol, are moderate inhibitors of arginase. Herein, we report our efforts to investigate catechol binding by quantum chemistry and generate analogues of piceatannol. In this work, we synthesized a novel series of amino-polyphenols which were then evaluated as arginase inhibitors. Their structure–activity relationships were elucidated by deep quantum chemistry modelling. 4-((3,4-Dihydroxybenzyl)amino)benzene-1,2-diol 3t displays a mixed inhibition activity on bovine and human arginase I with IC50 (Ki) values of 76 (82) μM and 89 μM, respectively.
- This article is part of the themed collection: Neglected Tropical Diseases


 Please wait while we load your content...
Please wait while we load your content...