Cyclic boronates as versatile scaffolds for KPC-2 β-lactamase inhibition†
Abstract
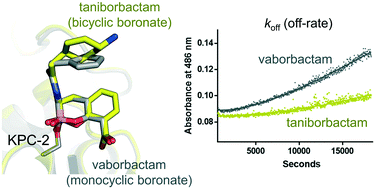
Klebsiella pneumoniae carbapenemase-2 (KPC-2) is a serine-β-lactamase (SBL) capable of hydrolysing almost all β-lactam antibiotics. We compare KPC-2 inhibition by vaborbactam, a clinically-approved monocyclic boronate, and VNRX-5133 (taniborbactam), a bicyclic boronate in late-stage clinical development. Vaborbactam inhibition is slowly reversible, whereas taniborbactam has an off-rate indicating essentially irreversible complex formation and a 15-fold higher on-rate, although both potentiate β-lactam activity against KPC-2-expressing K. pneumoniae. High resolution X-ray crystal structures reveal closely related binding modes for both inhibitors to KPC-2, with differences apparent only in positioning of the endocyclic boronate ester oxygen. The results indicate the bicyclic boronate scaffold as both an efficient, long-lasting, KPC-2 inhibitor and capable of supporting further iterations that may improve potency against specific enzyme targets and pre-empt the emergence of inhibitor resistant KPC-2 variants.


 Please wait while we load your content...
Please wait while we load your content...