Discovery of a novel kinase hinge binder fragment by dynamic undocking
Abstract
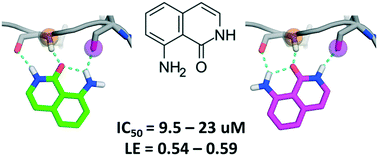
One of the key motifs of type I kinase inhibitors is their interactions with the hinge region of ATP binding sites. These interactions contribute significantly to the potency of the inhibitors; however, only a tiny fraction of the available chemical space has been explored with kinase inhibitors reported in the last twenty years. This paper describes a workflow utilizing docking with rDock and dynamic undocking (DUck) for the virtual screening of fragment libraries in order to identify fragments that bind to the kinase hinge region. We have identified 8-amino-2H-isoquinolin-1-one (MR1), a novel and potent hinge binding fragment, which was experimentally tested on a diverse set of kinases, and is hereby suggested for future fragment growing or merging efforts against various kinases, particularly MELK. Direct binding of MR1 to MELK was confirmed by STD-NMR, and its binding to the ATP-pocket was confirmed by a new competitive binding assay based on microscale thermophoresis.


 Please wait while we load your content...
Please wait while we load your content...