Protein modification by thiolactone homocysteine chemistry: a multifunctionalized human serum albumin theranostic†
Abstract
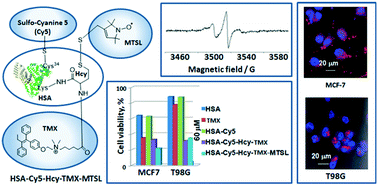
As the most abundant protein with a variety of physiological functions, albumin has been used extensively for the delivery of therapeutic molecules. Thiolactone chemistry provides a powerful tool to prepare spin-labeled albumin-based multimodal imaging probes and therapeutic agents. We report the synthesis of a tamoxifen homocysteine thiolactone derivative and its use in thiol-‘click’ chemistry to prepare multi-functionalized serum albumin. The released sulfhydryl group of the homocysteine functional handle was labeled with a nitroxide reagent to prepare a spin-labeled albumin–tamoxifen conjugate confirmed by MALDI-TOF-MS, EPR spectroscopy, UV-vis and fluorescent emission spectra. This is the basis for a novel multimodal tamoxifen–albumin theranostic with a significant (dose-dependent) inhibitory effect on the proliferation of malignant cells. The response of human glioblastoma multiforme T98G cells and breast cancer MCF-7 cells to tamoxifen and its albumin conjugates was different in tumor cells with different expression level of ERα in our experiments. These results provide further impetus to develop a serum protein for delivery of tamoxifen to cancer cells.


 Please wait while we load your content...
Please wait while we load your content...