Insights into structural and physicochemical properties required for β-hematin inhibition of privileged triarylimidazoles
Abstract
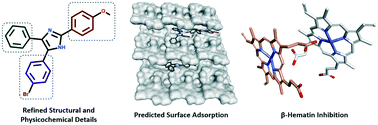
In this study, we investigated a series of triarylimidazoles, in an effort to elucidate critical SAR information pertaining to their anti-plasmodial and β-hematin inhibitory activity. Our results showed that in addition to the positional effects of ring substitution, subtle changes to lipophilicity and imidazole ionisability were important factors in SAR interpretation. Finally, in silico adsorption analysis indicated that these compounds exert their effect by inhibiting β-hematin crystal growth at the fast growing 001 face.


 Please wait while we load your content...
Please wait while we load your content...