Cu(i) diimine complexes as immobilised antibacterial photosensitisers operating in water under visible light†
Abstract
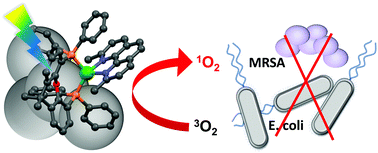
A complex of an Earth-abundant metal, copper, immobilised on silica offers a remarkably efficient way to kill bacteria in water under visible light, the first example of lighter transition metal complexes to do so. Photosensitisers which produce reactive oxygen species under light are emerging as an efficient way to kill microorganisms in water, yet the majority of photosensitising metal complexes are based on rare transition metals. Moreover, the efficiency of most solution-based photosensitisers is greatly compromised upon immobilisation on solid support, which is essential for safe treatment. Photosensitisers based on inexpensive metal complexes, such as those of Cu, Ni or Fe, usually have too short excited state lifetime to react efficiently with oxygen and are ineffective in production of reactive oxygen species. Here, we demonstrate that complexes of Cu(I) can be used as efficient photosensitisers for killing bacteria in water under visible light, when immobilised on surfaces, using as an example of [Cu(I)(xantphos)(dmp)]tfpb (1) [xantphos = 4,5-bis(diphenylphosphino)-9,9-dimethyl-xanthine, dmp = 2,9-dimethyl-1,10-phenanthroline, and tfpb = tetrakis(3,5-bis-(trifluoromethyl)-phenyl)borate] immobilised on silica, 1-silica. In contrast to many short-lived Cu(I) complexes, a sterically-hindered coordination centre in 1 leads to a relatively long excited state lifetime of >200 ns, which enables 1 to efficiently photosensitise singlet oxygen (29%). Upon irradiation, 1-silica (55 μM) shows high antibacterial activity against both a Gram-negative bacterium E. coli, and a Gram-positive bacterium Staphylococcus aureus (S. aureus, the Methicillin Resistant strain MRSA 315), both of which commonly occur in water. 99.99% killing of S. aureus was observed after only 15 min of irradiation with 17.5 mW cm−2 light, with 99.9999% (‘complete’) killing achieved after 2 h. For E. coli, 99.99% killing was achieved after 2 h, and 99.9999% after 3 h of irradiation. Thus 1-silica exceeds the ≥99.99% threshold set by WHO for the “highly protective” antibacterial agents. This first example of an immobilised Cu(I) complex used for light-driven bacterial killing demonstrates the potential of Earth-abundant transition metal complexes as low-cost efficient photo-antibacterial agents.
- This article is part of the themed collection: Celebrating Materials Science in the UK


 Please wait while we load your content...
Please wait while we load your content...