A biocompatible ZnNa2-based metal–organic framework with high ibuprofen, nitric oxide and metal uptake capacity†
Abstract
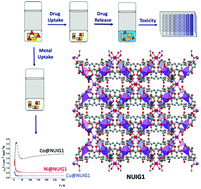
Metal organic frameworks (MOFs) have received significant attention in recent years in the areas of biomedical and environmental applications. Among them, mixed metal MOFs, although promising, are relatively few in number in comparison with their homometallic analogues. The employment of benzophenone-4,4′-dicarboxylic acid (bphdcH2) in mixed metal MOF chemistry provided access to a 3D MOF, [Na2Zn(bphdc)2(DMF)2]n (NUIG1). NUIG1 displays a new topology and is a rare example of a mixed metal MOF based on 1D rod secondary building units. UV-vis, HPLC, TGA, XRPD, solid state NMR and computational studies indicated that NUIG1 exhibits an exceptionally high Ibuprofen (Ibu) and nitric oxide adsorption capacity. The MCF-7 cell line was used to assess the toxicity of NUIG1 and Ibu@NUIG1, revealing that both species are non-toxic (cell viability > 70%). NUIG1 exhibits good performance in the adsorption of metal ions (CoII, NiII, CuII) from aqueous environments, as was demonstrated by UV-vis, EDX, ICP, SEM and direct and alternate current magnetic susceptibility studies. The colour and the magnetic properties of the M@NUIG1 species depend strongly on the kind and the amount of the encapsulated metal ion in the MOF pores.
- This article is part of the themed collections: Materials Advances HOT Article Collection and International Open Access Week 2020


 Please wait while we load your content...
Please wait while we load your content...