Biogenic synthesis of a silver nanoparticle–SnZrMoP nanocomposite and its application for the disinfection and detoxification of water
Abstract
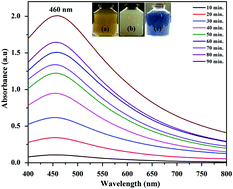
A silver nanoparticle (AgNP)–tin zirconium(IV) molybdophosphate (SnZrMoP) nano-composite was synthesized by a superficial and green synthetic approach using mulberry leaf extract, with the plant extract playing the role of a reducing agent as well as a capping agent. The nanocomposite was characterized by Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), and thermogravimetric analysis (TGA). The chemical stability of the nanocomposite and the effect of calcination on the ion-exchange capacity of the nanocomposite were also investigated. The nanocomposite was observed to be highly selective for Ba2+ and Sr2+ ions on the basis of distribution coefficient studies. The applications of the nanocomposite as an antimicrobial agent and for the quantitative and selective separation of toxic Ba2+ and Sr2+ ions from industrial effluents were explored.


 Please wait while we load your content...
Please wait while we load your content...