Metal organic framework-derived porous Fe2N nanocubes by rapid-nitridation for efficient photocatalytic hydrogen evolution†
Abstract
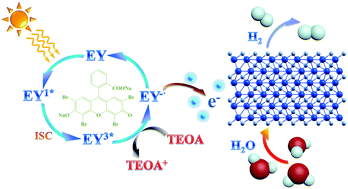
Transition metal nitrides are promising substitutes for noble metal catalysts in photocatalytic hydrogen evolution reaction. Here, we use the cubic metal organic framework (MOF) Prussian blue, which has been rarely explored for nitride preparation. A rapid nitridation process is used for obtaining porous iron nitride (Fe2N) nanoparticles. The phase-pure Fe2N catalyst reported largely maintains the cubic geometry and specific surface area of the parent MOF. Fe2N is sensitized using Eosin-Y for boosting photocatalytic hydrogen evolution. Hydrogen production rates close to ∼14.5 mmol g−1 h−1 are observed, which are considered desirable. This is reasonable since the nitride phase has high electronic conductivity and low electrocatalytic H2-generation overpotential. Calculations indicate that the electronic conductivity of Fe2N is due to Fe-3d states. This work opens up new possibilities for the production of porous nitride photocatalysts for efficient hydrogen evolution.


 Please wait while we load your content...
Please wait while we load your content...