Modeling ascending infection with a feto-maternal interface organ-on-chip†
Abstract
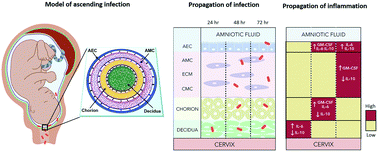
Maternal infection (i.e., ascending infection) and the resulting host inflammatory response are risk factors associated with spontaneous preterm birth (PTB), a major pregnancy complication. However, the path of infection and its propagation from the maternal side to the fetal side have been difficult to study due to the lack of appropriate in vitro models and limitations of animal models. A better understanding of the propagation kinetics of infectious agents and development of the host inflammatory response at the feto-maternal (amniochorion-decidua, respectively) interface (FMi) is critical in curtailing host inflammatory responses that can lead to PTB. To model ascending infection and determine inflammatory responses at the FMi, we developed a microfluidic organ-on-chip (OOC) device containing primary cells from the FMi (decidua, chorion, and amnion [mesenchyme and epithelium]) and collagen matrix harvested from primary tissue. The FMi-OOC is composed of four concentric circular cell/collagen chambers designed to mimic the thickness and cell density of the FMi in vivo. Each layer is connected by arrays of microchannels filled with type IV collagen to recreate the basement membrane of the amniochorion. Cellular characteristics (viability, morphology, production of nascent collagen, cellular transitions, and migration) in the OOC were similar to those seen in utero, validating the physiological relevance and utility of the developed FMi-OOC. The ascending infection model of the FMi-OOC, triggered by exposing the maternal (decidua) side of the OOC to lipopolysaccharide (LPS, 100 ng mL−1), shows that LPS propagated through the chorion, amnion mesenchyme, and reached the fetal amnion within 72 h. LPS induced time-dependent and cell-type-specific pro-inflammatory cytokine production (24 h decidua: IL-6, 48 h chorion: GM-CSF and IL-6, and 72 h amnion mesenchyme and epithelium: GM-CSF and IL-6). Collectively, this OOC model and study successfully modeled ascending infection, its propagation, and distinct inflammatory response at the FMi indicative of pathologic pathways of PTB. This OOC model provides a novel platform to study physiological and pathological cell status at the FMi, and is expected to have broad utility in the field of obstetrics.


 Please wait while we load your content...
Please wait while we load your content...