Direct analysis of liquid drugs by inductively coupled plasma mass spectrometry using aerosol dilution†
Abstract
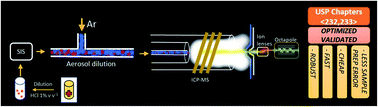
In this work a method for the direct analysis of liquid drug samples by inductively coupled plasma mass spectrometry (ICP-MS) with aerosol dilution was proposed. Multivariate studies were executed for optimization of ICP-MS parameters (sample depth, aerosol dilution gas flow rate and He gas flow rate) using a representative diluted drug sample aiming at appropriate plasma robustness parameters (140Ce2+/140Ce+ and 140Ce16O+/140Ce+ ratios), as well as sensitivity for Ag, As, Au, Ba, Cd, Co, Cr, Cu, Hg, Ir, Li, Mo, Ni, Os, Pb, Pd, Pt, Rh, Ru, Sb, Se, Sn, Tl and V, the elemental impurities in USP Chapter 232. The results showed that the dilution gas had a major role in formation of doubly charged and oxide ions. A robust plasma condition was achieved (i.e., 140Ce2+/140Ce+ < 0.9% and 140Ce16O+/140Ce+ < 0.3%) when 0.40 L min−1 dilution gas flow rate was used. The combination of collision (He) gas and dilution gas flow rates was the key feature for minimizing (or even removing) polyatomic interferences. It was verified that collision cell processes benefit from less sample matrix introduction provided by aerosol dilution. Method validation has been accomplished and reported for a liquid drug for hepatic disorders. The results successfully met the requirements of the USP Chapter 233 procedure, wherein appropriate figures of merit were obtained (R > 0.995 and RSD < 6.2% for precision tests, RSD < 14% for intermediate precision tests, and 88–125% recoveries for a 50-fold diluted spiked sample with different J concentrations). This study provided useful information about the benefits of aerosol dilution and demonstrates that ICP-MS with aerosol dilution is a reliable method for the direct analysis of liquid drug samples in order to meet the requirements of USP Chapters 232 and 233.


 Please wait while we load your content...
Please wait while we load your content...