Nitromethane as a surrogate cyanating agent: 7-N,N-dimethylamino-4-hydroxycoumarin-catalyzed, metal-free synthesis of α-iminonitriles†
Abstract
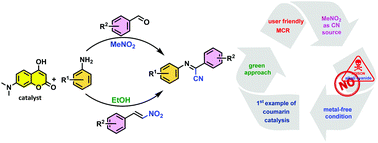
An efficient, metal/alkali-cyanide free approach for the synthesis of α-iminonitriles via kinetically controlled, base-mediated and 1,3-diketone-catalyzed reaction is reported. The preparation of target compounds was realized by condensation of substituted anilines and aldehydes in nitromethane as a surrogate cyanating agent and as a solvent. This strategy was further improved by replacing aldehydes and nitromethane with β-nitrostyrene and ethanol, respectively, rendering the methodology greener. The catalytic role played by 1,3-diketones such as 7-N,N-dimethylamino-4-hydroxycoumarin in this three-component reaction was investigated, and a plausible mechanism was proposed based on control experiments.
- This article is part of the themed collection: 2020 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...