Unraveling the cation and anion effects and kinetics for ionic liquid catalyzed direct synthesis of methyl acrylate under mild conditions†
Abstract
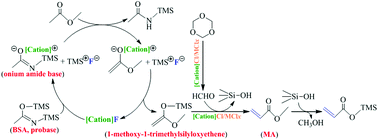
The direct synthesis of methyl acrylate (MA) from methyl acetate and trioxane at 350–380 °C is regarded as a supplementary route for the industrial propylene oxidation process; however, it suffers from rapid catalyst deactivation. Herein, a novel ionic liquid catalyzed mild liquid-phase system was developed for the direct synthesis of MA from methyl acetate and trioxane, where N,O-bis(trimethylsilyl)acetamide (BSA) was used as a probase for α-deprotonation and enol silyl etherification of methyl acetate. The trioxane decomposition to formaldehyde and methyl acetate enolization to 1-methoxy-1-trimethylsilyloxyethene proceeded with the catalysis of [Cation]Cl/MClx (M = Cu+, Fe3+, Zn2+ and Al3+) and [Cation]F, respectively. The cations and anions were observed to have significant effects on the yield and selectivity of MA, owing to the steric hindrance, acid site category and strength confirmed by pyridine probing FT-IR characterization. As a result, up to 60.2% yield with 94.6% selectivity of MA could be achieved when [N3,3,3,3]F and [N3,3,3,3]Cl/AlCl3 with 67 mol% AlCl3 were used in the presence of BSA at 25 °C. Kinetic studies indicated that the trioxane decomposition with the activation barrier of 41.2 ± 0.3 kJ mol−1 was the rate-determining step.


 Please wait while we load your content...
Please wait while we load your content...