Synthesis and self-assembly of aminyl and alkynyl substituted sophorolipids†
Abstract
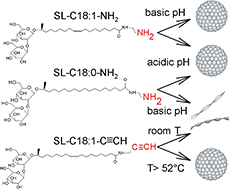
Sophorolipids are one of the most important microbial biosurfactants, because of their large-scale production and applications developed so far in the fields of detergency, microbiology, cosmetics or environmental science. However, the structural variety of native sophorolipids is limited/restricted, a limiting fact for the development of new properties and their potential applications. In their open acidic form, C18:1 sophorolipids (SL) are classically composed of a sophorose headgroup and a carboxylic acid (COOH) end-group. The carboxyl group gives them unique pH-responsive properties, but it is a poorly reactive group and its charge can only be negative. To develop a new generation of pH-responsive, positively charged, SL and to improve their reactivity for further functionalization, we develop here SL with an amine (–NH2) or terminal alkyne (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) end-group analogues. The amine group generates positively charged SL and is more reactive than carboxylic acids, e.g. towards aldehydes; the alkyne group provides access to copper-based click chemistry. In this work, we synthesized (C18:1) and (C18:0) –NH2 and (C18:1) –C
CH) end-group analogues. The amine group generates positively charged SL and is more reactive than carboxylic acids, e.g. towards aldehydes; the alkyne group provides access to copper-based click chemistry. In this work, we synthesized (C18:1) and (C18:0) –NH2 and (C18:1) –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH sophorolipid derivatives and we study their self-assembly properties in response to pH and/or temperature changes by means of static and dynamic light scattering, small angle (X-ray, neutron) scattering and cryogenic electron microscopy. Monounsaturated aminyl SL-C18:1-NH2 sophorolipids form a micellar phase in their neutral form at high pH and a mixed micellar-bilayer phase in their positively charged form at low pH. Saturated aminyl SL-C18:0-NH2 sophorolipids form a micellar phase in their charged form at low pH and a twisted ribbon phase in their neutral form at high pH and monounsaturated alkynyl SL-C18:1-C
CH sophorolipid derivatives and we study their self-assembly properties in response to pH and/or temperature changes by means of static and dynamic light scattering, small angle (X-ray, neutron) scattering and cryogenic electron microscopy. Monounsaturated aminyl SL-C18:1-NH2 sophorolipids form a micellar phase in their neutral form at high pH and a mixed micellar-bilayer phase in their positively charged form at low pH. Saturated aminyl SL-C18:0-NH2 sophorolipids form a micellar phase in their charged form at low pH and a twisted ribbon phase in their neutral form at high pH and monounsaturated alkynyl SL-C18:1-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH sophorolipids form a main micellar phase at T > 51.8 °C and a twisted ribbon phase at T < 51.8 °C.
CH sophorolipids form a main micellar phase at T > 51.8 °C and a twisted ribbon phase at T < 51.8 °C.
- This article is part of the themed collection: 2020 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...