Concurrent thiol–ene competitive reactions provide reprocessable, degradable and creep-resistant dynamic–permanent hybrid covalent networks†
Abstract
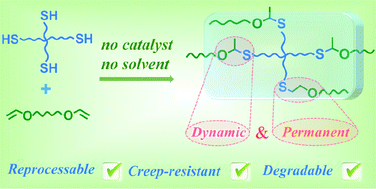
A catalyst-free, solvent-free thiol–ene addition reaction was developed to produce a dynamic–permanent hybrid cross-linked polymer from two simple components, a tetrathiol and a divinyl ether, in one pot. The tetrathiol and divinyl ether underwent two competitive Markovnikov and anti-Markovnikov addition reactions simultaneously and separately generated S,O-thioacetal cross-links and thioether cross-links. The dynamic feature of S,O-thioacetal cross-links imparts excellent malleability, reprocessability and degradability to the obtained network, and the permanent covalent thioether cross-links provide favorable high-temperature creep resistance. This work will broaden the thiol–ene chemistry and provide a green method to achieve recyclable cross-linked polymers and covalent adaptable networks (CANs) or vitrimer-like materials with high-temperature creep resistance.


 Please wait while we load your content...
Please wait while we load your content...