Biaryl and atropisomeric biaryl aldehyde synthesis by one-step, metal-free benzannulation of aryl enals and propiolates†
Abstract
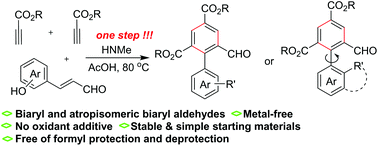
A new method involving the benzannulation of aromatic enals and two alkyl propiolate molecules has been developed as a powerful route to biaryl aldehydes simply via the promotion of dimethyl amine. The benzannulation process in the absence of an oxidant additive tolerates successfully the formyl group in the enal component, leading to a straightforward one-step synthesis of biaryl and atropisomeric aldehydes. An enamine activation based on the aza-Michael addition of dimethyl amine to the propiolate and the amine elimination-based generation of cyclohexadiene intermediate constitute the major factors enabling the titled reactions.
- This article is part of the themed collection: 2020 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...