Expeditious and sustainable two-step synthesis of sinapoyl-l-malate and analogues: towards non-endocrine disruptive bio-based and water-soluble bioactive compounds†
Abstract
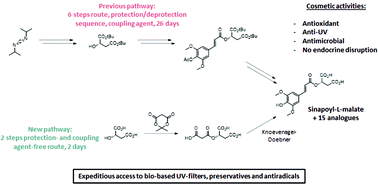
Faced with the increasing demand from both the cosmetic industries and consumers for bio-based, safe and natural skin products, sinapoyl-L-malate, widely described for its UV protection in plants, appears to be an excellent alternative to substitute chemical filters in sunscreens. Unfortunately, the only synthetic routes described in the literature were not only tedious but also exhibit a strong negative environmental impact, thus seriously limiting the industrialization and commercialization of sinapoyl-L-malate. Herein, a shorter and greener synthetic pathway involving Meldrum's acid opening with unprotected naturally occurring hydroxy acids and its subsequent Knoevenagel-Doebner condensation with biomass-derived p-hydroxybenzaldehydes was designed and developed. This two-step procedure, whom sustainability has been assessed using green metrics (atom economy (AE), process atom economy (PAE), E-factor and LCA), is a great alternative to the already reported procedures and allows the access to sinapoyl-L-malate and several analogs in average to good yield. The study of the anti-UV properties, stability against UV radiation, radical scavenging and antimicrobial activities of the targets revealed attractive properties as photostable UV filters, antioxidants and preservatives. Moreover, the water solubility brought by the free carboxylic acids facilitates the incorporation of these molecules in cosmetic formulations. Finally, their innocuousness toward endocrine disruption was demonstrated.


 Please wait while we load your content...
Please wait while we load your content...