Highly efficient production of lactic acid from xylose using Sn-beta catalysts†
Abstract
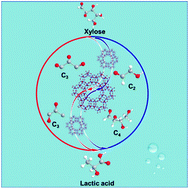
The efficient conversion of xylose into lactic acid, especially with the novel contribution of C2 components, was revealed over the heterogeneous Sn-beta catalyst in water with a very high lactic acid yield of 70.0 wt% at 200 °C for 60 min. The 13C NMR results indicated that glycolaldehyde (C2), the cleavage species of xylose condensate to erythrose (C4), subsequently, erythrose converts to lactic acid (C3) and to formic acid (C1) with the removal of a carbon atom. In this catalytic process, Sn acts as the Lewis acid site in the Si–O–Sn framework, and participates in the coupling and cracking of C–C bonds (C2 → C4 → C3) through the adsorption of α-protons to generate carbonium anions. Thus, more than 10 wt% lactic acid was obtained based on above pathway through the synergy of aldol addition, isomerization and retro-aldol condensation over the Sn-beta catalyst.
- This article is part of the themed collection: 2020 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...