Tuning selectivity of CO2 hydrogenation by modulating the strong metal–support interaction over Ir/TiO2 catalysts†
Abstract
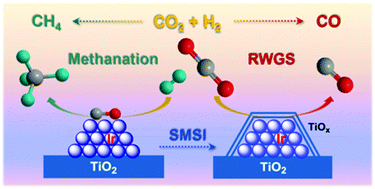
Exploration of highly selective catalysts for CO2 hydrogenation remains a great challenge since the reduction of CO2 over the supported metal catalysts may give rise to various products in response to the modulation of the chemical state of active sites. Herein, by varying the pretreatment temperature of iridium/titanium oxide (Ir/TiO2) catalysts, the selectivity of CO2 hydrogenation from CH4 to sole production of CO can be finely tuned. The change of product selectivity is achieved in such a way that the selectivity greatly depend on the formation of a reduced TiOx overlayer around Ir nanoparticles (NPs) as originated from the strong metal–support interaction (SMSI). With only a weak reduction treatment, the exposed Ir NPs without a TiOx coating promote CH4 production exclusively. After the catalyst undergoes a high temperature reduction, the evolution of the TiOx coating over Ir NPs shows a preference for CO production with an inhibition of further methanation. This study not only provides insights into the regulation of CO2 hydrogenation by SMSI, but also serves as an effective approach to tuning other catalytic processes.
- This article is part of the themed collection: CO2 Utilisation


 Please wait while we load your content...
Please wait while we load your content...