A unique pathway to platform chemicals: aldaric acids as stable intermediates for the synthesis of furandicarboxylic acid esters†
Abstract
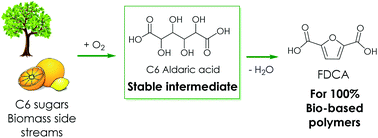
2,5-Furandicarboxylic acid (FDCA) has received attention as an emerging bio-based building block with many applications, especially in renewable polyesters. The common route to FDCA uses the unstable 5-hydroxymethylfurfural (HMF) as an intermediate. Here, we present an alternative route to FDCA and its esters using C6 aldaric acids as stable intermediates. Aldaric acids, or sugar diacids, can be obtained by the oxidation of C6 sugars or uronic acids from pectin. Subsequent dehydration of aldaric acids by solid acid catalysts in butanol produces furancarboxylates. Using silica-supported acid catalysts, over 90% yields of furancarboxylates were achieved with the selectivity to FDCA and its esters reaching 80%.


 Please wait while we load your content...
Please wait while we load your content...