Modulating trans-imination and hydrogenation towards the highly selective production of primary diamines from dialdehydes†
Abstract
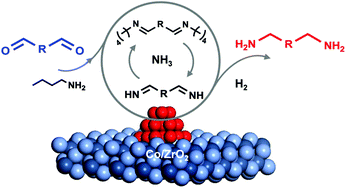
Bio-based primary diamines are important building blocks for sustainable bio-polymers, but their synthesis remains challenging due to the high susceptibility to polymerization. Herein, we have developed a new strategy to suppress the polymerization by employing a more nucleophilic alkylamine to scavenge the dialdehyde and a Co/ZrO2 catalyst to regulate the trans-imination and hydrogenation activity. With this strategy, 2,5-bis(aminomethyl)furan (BAMF), a promising monomer for the production of new polyamides and polyureas, is successfully synthesized via the reductive amination of biomass-derived 2,5-diformylfuran (DFF) under a H2 and NH3 atmosphere with an unprecedentedly high selectivity up to 95%. This strategy is applicable to the reductive amination of other biomass-derived dialdehydes, thus paving a new way to bio-based diamine monomers.


 Please wait while we load your content...
Please wait while we load your content...