Sulfite modification of platinum nanoparticles modulates electrocatalytic formic acid oxidation activity†
Abstract
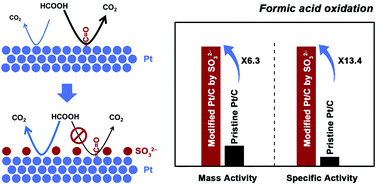
It is highly desirable to enhance the catalytic activities of noble metal nanoparticles, especially commercially available ones, by a simple post-synthetic treatment, which may become a convenient, cost-effective, and industrially viable strategy to produce high-performance catalysts for many important processes. Herein, we report a successful case with pre-existing Pt nanoparticles targeting a highly active catalyst for the electrocatalytic formic acid oxidation reaction (FAOR), the key anode reaction for direct formic acid fuel cells (DFAFCs), by adsorbing a small-molecule inorganic ligand of sulfite (SO32−) on the metal catalysts. The adsorption of sulfite affords isolated Pt sites that can effectively suppress the dehydration pathway of the FAOR and thereby minimize the evolution of poisonous CO as an intermediate. It further allows the convenient oxidation of CO possibly produced via the dehydration pathway at a much lower potential. Due to the concerted effects, the sulfite-modified commercial Pt/C showed a 13.4-fold increase in the specific activity and a 6.3-fold increase in the mass activity in the electrocatalytic FAOR, compared with the pristine catalyst. This strategy is easy to operate and scale up, free of toxic or organic compounds (thus environmentally friendly), and directly applicable to already existing commercial catalysts, which opens an attractive route to the tailoring of catalytic properties of noble metal catalysts for DFAFCs and many other applications.


 Please wait while we load your content...
Please wait while we load your content...